To become a global well-known life
science company
Miconvey, focusing on its research and innovation, is an ambitious and reliable medical technology corporate located in Chongqing, China. Since its founding in 2013, Miconvey has made it a priority to develop and manufacture minimally invasive surgical instruments with high quality beyond the level of peer companies.
Trusted by clients from all over the world, Miconvey continues to strengthen the core competitiveness of its goods with an open and innovative R&D methodology, taking advantage of its strong BD capabilities and senior international expert team.
History
Our Strength
Engineering Excellence
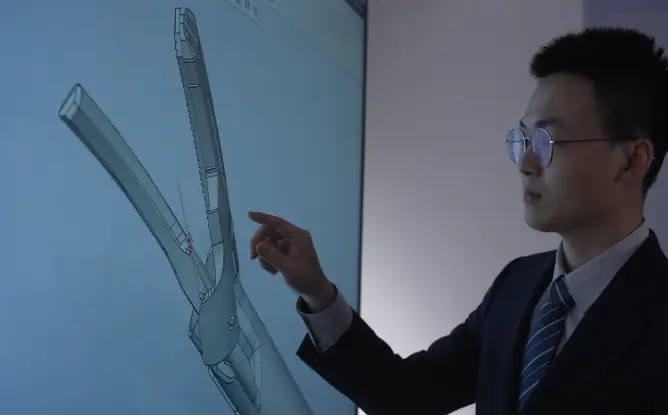
Our creativity stems from our advanced production technologies. As a pioneer of minimally invasive surgical instrument platforms in China, Miconvey possesses multiple core independent intellectual properties. In addition, Miconvey has continuously upgraded its products to compete in the global market and lead technological innovations in the industry as a pioneer.
Miconvey has more than 100 patent applications at home and abroad. Up to now, it has been granted 82 patents, including 17 invention patents.
Miconvey has more than 100 patent applications at home and abroad. Up to now, it has been granted 82 patents, including 17 invention patents.
Manufacturing Excellence
Our core competitiveness lies in lean manufacturing. By giving priority to product quality, we meticulously control every manufacturing link to ensure consistent quality.
We have established Class 100,000 cleanrooms dedicated to the production of medical instruments and consumables, as well as large-scale workshops equipped with intelligent CNC manufacturing equipment, to maintain our leading position in medical technology and align with international standards.
We have established Class 100,000 cleanrooms dedicated to the production of medical instruments and consumables, as well as large-scale workshops equipped with intelligent CNC manufacturing equipment, to maintain our leading position in medical technology and align with international standards.
Quality Control
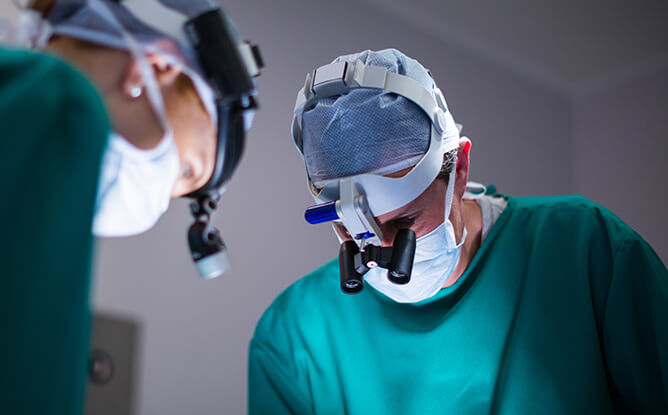
Miconvey adheres to strict quality processes to guarantee the performance and durability of all surgical instruments. And we employ a qualified team to oversee quality control, specifically the techniques and activities aimed at meeting the quality requirements.
Each piece of Miconvey equipment is closely examined, and if there is a slight blemish on it, it is not for sale.
Each piece of Miconvey equipment is closely examined, and if there is a slight blemish on it, it is not for sale.
All efforts for health
Upholding the core values of “Aspiration, Striving, Innovation, Inclusiveness and Integrity”, Miconvey will continuously pursue high-quality development and make unremitting efforts to become a globally well-known life science company.